KEYWORDS
Rhamnogalacturonan-I
RG-I
Prebiotic
Metabolites
Health
Abstract
Emerging evidence highlights the capacity of the gut microbiota to influence systemic health via systems referred to as "gut-health axes". Dietary supplementation with prebiotic fibers therefore offers a promising opportunity to support e.g., gut, brain, metabolic, and cardiovascular health, as well as healthy ageing. Carrot-derived rhamnogalacturonan-I (cRG-I) is a precision prebiotic fiber that selectively modulates gut microbiota composition and specifically boosts the microbial conversion of tryptophan to IPA and enhances the production of other key metabolites like SCFAs and GABA. The aim of this paper is to review the scientific evidence supporting some of the health benefits that could be affected by cRG-I-derived metabolites.
Abstract
Emerging evidence highlights the capacity of the gut microbiota to influence systemic health via systems referred to as "gut-health axes". Dietary supplementation with prebiotic fibers therefore offers a promising opportunity to support e.g., gut, brain, metabolic, and cardiovascular health, as well as healthy ageing. Carrot-derived rhamnogalacturonan-I (cRG-I) is a precision prebiotic fiber that selectively modulates gut microbiota composition and specifically boosts the microbial conversion of tryptophan to IPA and enhances the production of other key metabolites like SCFAs and GABA. The aim of this paper is to review the scientific evidence supporting some of the health benefits that could be affected by cRG-I-derived metabolites.
Introduction
“All disease starts in the gut,” claimed Hippocrates over 2,000 years ago. Today, science is catching up with this ancient insight, as we continue to unravel the complex and dynamic relationship between the gut microbiota, health, and disease. One of the most promising developments in this field is the recognition of microbially-derived metabolites as key contributors to health and wellbeing.
Supporting a healthy and diverse gut microbiota has been linked to numerous health benefits—ranging from stronger intestinal barrier integrity and enhanced resilience against pathogens to a reduced risk of chronic diseases (1,2,3). A crucial factor in maintaining this microbial diversity is the availability of fermentable substrates—specifically, prebiotic fibers that serve as nourishment for beneficial gut microbes. With inadequate fiber intake, we risk a decline in microbial diversity, a loss of key bacterial species, and reduced production of health-promoting metabolites (4,5).
Carrot-derived rhamnogalacturonan-I (cRG-I) is a precision prebiotic fiber that can selectively modulate the composition and function of the gut microbiota. By doing so, it represents an exciting approach for supporting health through the gut.
Despite widespread awareness that a balanced diet should include around 30 grams of fiber per day—ideally from a variety of sources, including prebiotics—fiber intake in Western countries remains consistently low. In the United States, for instance, up to 95% of the population fails to meet the recommended daily intake (6). Dietary supplementation with prebiotic fibers therefore represents a simple way to increase overall fiber intake, and subsequently modulate the composition of the gut microbiota as well as its activity.
Recent research demonstrates that prebiotic fibers differ in their physiological effects, with certain fiber combinations exhibiting synergistic activity (7,8). Notably, some fibers—such as cRG-I—have been shown to confer gut and immune health benefits even at low doses (9,10,11)
Multiple Benefits Through Gut-Health Axes
The gastrointestinal (GI) tract is not only responsible for the digestion and absorption of nutrients, but also serves as a critical barrier between our internal environment and the external world. It is also the body’s largest immune organ, and harbours a complex microbial ecosystem—the gut microbiota—which plays a central role in maintaining gut health.
Emerging evidence highlights the capacity of the gut microbiota to influence systemic health via bi- or multi-directional communication along neural, immune, endocrine, and metabolic pathways. Examples of these interconnected systems, often referred to as "gut-health axes," are illustrated in Figure 1.
Targeting the gut microbiota through selective nutritional strategies offers a promising approach to modulate these gut-health axes. For instance, the use of prebiotic fibers such as cRG-I to promote the growth of specific health-associated bacteria and enhance the production of bioactive microbial metabolites represents a compelling approach to influence multiple health outcomes.
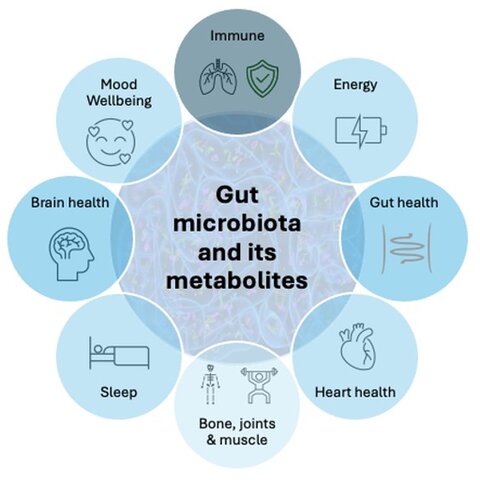
Figure 1. Some health benefits that can be modulated by the gut microbiota and its metabolites.
cRG-I enhances the production of beneficial microbial metabolites
Fermentation of cRG-I by the gut microbiota has been shown to significantly increase the abundance of beneficial microorganisms such as bifidobacteria and anti-inflammatory bacterial species. It also results in the production of short chain fatty acids (SCFA) (9,12). More recently, the fermentation of cRG-I was also shown to consistently increase the production of other potentially health promoting metabolites (13). Table 1 shows the metabolites that were significantly enhanced by cRG-I as well as their associated health benefits (13).
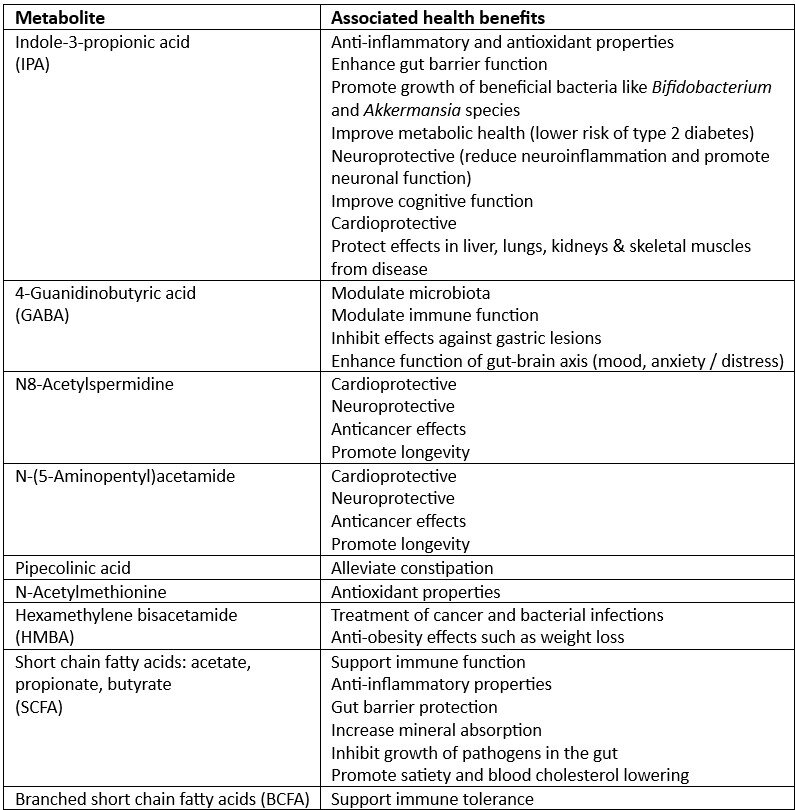
Table 1. cRG-I significantly enhanced the microbial production of health-related metabolites.
Mapping of metabolites to health benefits
To explore the health relevance of key gut-derived metabolites, we used mined insights from scientific literature by leveraging a biological interpretation platform. We screened over 35 million PubMed abstracts using a curated set of more than 500,000 keywords—covering a wide spectrum of biological concepts including metabolites, pathways, microbial species, cell types, and diseases. This analysis generated a KMINE report which was visualized as a heatmap and contained both literature abstracts and statements filtered for the most meaningful connections between metabolites and health outcomes (https://www.tenwise.nl).
Potential Health Benefits of cRG-I
A diverse and balanced gut microbiota is fundamental to maintaining health. Gut dysbiosis—defined as a disruption in the composition and function of the gut microbial community—has been associated with an increased risk of both intestinal and extra-intestinal conditions (3). In addition to its established benefits for gut and immune health, emerging evidence from the scientific literature suggests that cRG-I may confer broader health benefits via multiple gut-health axes (9,10,11).
Gut Health
The gut microbiota contributes to host health through several key mechanisms including the fermentation of complex carbohydrates, synthesis of essential vitamins, modulation of immune responses, prevention of pathogen colonization and production of a variety of bioactive compounds.
While the gut microbiota composition is relatively resilient to short-term perturbations, persistent stressors such as poor diet, chronic inflammation, or antibiotic use can induce dysbiosis. Importantly, dysbiosis which has been implicated in the pathogenesis and pathophysiology of gastrointestinal disorders like irritable bowel syndrome (IBS) (14) and inflammatory bowel disease (IBD) (3,15), not only alters microbial composition but can also impair functional outputs, e.g., the biosynthesis of health-promoting metabolites. For example, reduced levels of indole-3-propionic acid (IPA), a microbial metabolite derived from tryptophan, have been observed in the feces of individuals with IBD (16).
The fermentation of cRG-I by gut microbiota has been shown to increase levels of IPA and short-chain fatty acids (SCFAs), both of which exhibit anti-inflammatory properties and contribute to the maintenance of gut barrier function (12,13). In addition, the microbial production of γ-aminobutyric acid (GABA) has been linked to enhanced epithelial barrier integrity through upregulation of tight junction proteins and stimulation of mucus production (17).
Since intestinal barrier dysfunction—commonly referred to as “leaky gut”—is increasingly recognized as a critical factor in the etiology of immune, neuropsychological and metabolic disorders, reinforcing gut barrier integrity is essential for gut health and broader physiological resilience and wellbeing (18,19,20,21).
Brain health, mood and wellbeing
The gut-brain axis describes bidirectional communication between the gut microbiota and the brain, involving complex interactions across the gastrointestinal, immune, and central nervous systems (CNS). Disruptions in this axis have been linked to the development and progression of various psychiatric (e.g., depression, anxiety, mood disorders) and neurological conditions (e.g., autism, Parkinson’s disease, multiple sclerosis, and chronic pain) (18,19).
Interestingly, the gut microbiota can contribute to the production of neuroactive molecules that can influence brain function (22). For example, reduced levels of the inhibitory neurotransmitter GABA, have been associated with anxiety and depression (23).
Fermentation of the precision prebiotic cRG-I has been shown to increase the production of several metabolites with potential benefits for brain health, mood, and overall wellbeing (13). These include GABA, SCFAs, IPA, N8-acetylspermidine and N-(5-aminopentyl) acetamide. SCFAs and IPA, are thought to support CNS function by modulating the mucosal immune system and signaling through enteroendocrine and enterochromaffin cells in the gut (18). IPA produced in the gut can enter the systemic circulation and cross the blood-brain barrier, where it can reduce neuro-inflammation, scavenge reactive oxygen species, protect neurons from oxidative stress, and prevent neuro-degeneration (24). Furthermore, IPA has been reported to enhance nerve regeneration and sensory recovery by promoting neutrophil chemotaxis to dorsal root ganglion neurons, and to modulate microglial activation within the central nervous system (25,26). Elevated levels of N8-acetylspermidine and N-(5-aminopentyl) acetamide metabolites have been associated with neuroprotective effects and implicated in the regulation of brain function and cognition (13,27).
Metabolic and Cardiovascular Health
The gut microbiota plays a pivotal role in metabolic regulation, and disturbances in its composition are increasingly linked to the pathogenesis of metabolic disorders such as diabetes and obesity (28,29). These disorders are well-established risk factors for cardiovascular disease.
The fermentation of cRG-I by gut microbiota leads to an increase in the production of SCFAs, notably propionate, which has been implicated in the regulation of several metabolic processes, including insulin secretion, lipolysis, and the production of leptin and glucagon-like peptide-1 (GLP-1) (30). Fermentation of cRG-I also enhances IPA production. Circulating IPA levels have been inversely associated with key parameters of metabolic syndrome and reduced serum IPA concentrations have been observed in individuals with type 2 diabetes and non-alcoholic fatty liver disease (NAFLD), as well as in patients with atherosclerosis (31,32,33).
In addition, cRG-I fermentation increases levels of N8-acetylspermidine and N-(5-aminopentyl)acetamide (also known as N-acetylcadaverine), which have been associated with cardioprotective effects in preclinical studies (27). Another metabolite elevated by cRG-I fermentation is hexamethylene bisacetamide (HMBA), which has been associated with anti-obesity effects in preclinical models (34).
Respiratory Health
The gut-lung axis refers to the bidirectional communication between the gastrointestinal and respiratory systems, mediated by the microbiota, its metabolites, and interactions with the immune, neural, and endocrine systems. For example, gut-resident microbes can modulate respiratory immunity by producing microbial metabolites—like SCFAs and IPA— that can affect the airways or influence the migration of immune cells from the gut to the lungs (35).
Gut dysbiosis can result from respiratory infections, observed as e.g., a lower abundance of SCFA-producing bacteria in patients with respiratory tract infections (36,37). It can also result from antibiotic use, leading to lower production of IPA (38). Interestingly, gut dysbiosis has been linked to the development of respiratory diseases such as asthma (38,39).
Dietary supplementation with cRG-I has shown faster anti-viral responses to a rhinovirus infection which resulted in reduced severity and duration of symptoms as well as increased abundance of SCFA-producing bacteria (9,10,11,12). In addition, the fermentation of cRG-I leads to increased production of SCFAs, IPA and GABA, all metabolites that may act as immune modulators with anti-inflammatory effects in the airways (13).
Healthy Ageing
The gut microbiota plays a central role in the ageing process. Dysbiosis has been associated with increased gut permeability, impaired metabolic function, and the promotion of chronic low-grade inflammation ("inflammageing"), all of which are linked to age-related diseases including type 2 diabetes, cardiovascular disease, cognitive decline, and sarcopenia (40).
Fermentation of cRG-I leads to elevated levels of IPA, which has been shown to reshape the gut microbial composition and inhibit dysbiosis in preclinical studies (41). Additionally, cRG-I increases the production of N8-acetylspermidine and N-(5-aminopentyl)acetamide—metabolites associated with neuroprotective and cardioprotective functions—which may contribute to the maintenance of cognitive and cardiovascular health during ageing (27,42). SCFAs generated during cRG-I fermentation also support healthy ageing e.g., by modulating immune responses and regulating metabolism (43,44).
Reduction of Potentially Harmful Metabolites
Interestingly, cRG-I fermentation not only increases beneficial microbial metabolites but also reduces levels of compounds associated with adverse health effects. For instance, 12,13-dihydroxy-9Z-octadecenoic acid (12,13-DiHOME), which is reported to exhibit immunosuppressive activity and may impair the development of immune tolerance, is decreased following cRG-I fermentation. Similarly, 13-hydroxyoctadecadienoic acid (13-HODE)—a metabolite linked to asthma, hepatic steatosis, and certain cancers—is also reduced (45,46,47). This dual capacity to elevate protective and lower detrimental metabolites highlights the broad potential of cRG-I in supporting health across multiple gut-health axes.
Conclusions
Considering the growing body of evidence supporting the role of microbially-derived metabolites in promoting health, dietary supplementation with prebiotic fibers presents a promising opportunity to support gut, brain, metabolic, and cardiovascular health, as well as healthy ageing. This strategy may even help prevent or manage a variety of health conditions.
The precision prebiotic fiber cRG-I, which specifically boosts the microbial conversion of tryptophan to IPA and enhances the production of other key metabolites like SCFAs and GABA, stands out as a particularly attractive candidate for developing functional foods, beverages, or supplements. Effective at a remarkably low dose (9,10,11), cRG-I also offers practical advantages: (i) excellent tolerability without gastrointestinal discomfort, (ii) ease of incorporation into food and beverage formulations, and (iii) suitability for small-format dietary supplements.
More than 2000 years after Hippocrates’ insight, we are beginning to understand the profound influence of the gut on overall health—and, importantly, we now have tools to act on it. With the help of targeted dietary strategies—using prebiotics like cRG-I—we might not only say that all disease starts in the gut, but confidently propose that better health starts in the gut as well.
Conflicts of Interest: Sue McKay is an employee of NutriLeads BV and Wynand Alkema is co-owner and founder of TenWise.
References and notes
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445, doi:10.1016/s0140-6736(18)31809-9.
- Spragge, F.; Bakkeren, E.; Jahn, M.T.; Araujo, E.B.N.; Pearson, C.F.; Wang, X.; Pankhurst, L.; Cunrath, O.; Foster, K.R. Microbiome Diversity Protects against Pathogens by Nutrient Blocking. Science 2023, 382, eadj3502–eadj3502, doi:10.1126/science.adj3502.
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135, doi:10.1038/s41392-022-00974-4.
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715, doi:10.1016/j.chom.2018.05.012.
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786, doi:10.1016/j.cmet.2014.07.003.
- Thompson, H.J.; Brick, M.A. Perspective: Closing the Dietary Fiber Gap: An Ancient Solution for a 21st Century Problem. Adv. Nutr. 2016, 7, 623–626, doi:10.3945/an.115.009696.
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio 2021, 12, e01028-21, doi:10.1128/mbio.01028-21.
- Cantu-Jungles, T.M.; Agamennone, V.; Broek, T.J.V. den; Schuren, F.H.J.; Hamaker, B. Systematically-Designed Mixtures Outperform Single Fibers for Gut Microbiota Support. Gut Microbes 2025, 17, 2442521, doi:10.1080/19490976.2024.2442521.
- Jian, C.; Sorensen, N.; Lutter, R.; Albers, R.; Vos, W. de; Salonen, A.; Mercenier, A. The Impact of Daily Supplementation with Rhamnogalacturonan-I on the Gut Microbiota in Healthy Adults: A Randomized Controlled Trial. Biomed. Pharmacother. 2024, 174, 116561, doi:10.1016/j.biopha.2024.116561.
- Lutter, R.; Teitsma-Jansen, A.; Floris, E.; Lone-Latif, S.; Ravi, A.; Pineros, Y.S.S.; Dekker, T.; Smids, B.; Khurshid, R.; Aparicio-Vergara, M.; et al. The Dietary Intake of Carrot-Derived Rhamnogalacturonan-I Accelerates and Augments the Innate Immune and Anti-Viral Interferon Response to Rhinovirus Infection and Reduces Duration and Severity of Symptoms in Humans in a Randomized Trial. Nutrients 2021, 13, 4395, doi:10.3390/nu13124395.
- McKay, S.; Teitsma-Jansen, A.; Floris, E.; Dekker, T.; Smids, B.; Khurshid, R.; Calame, W.; Kardinaal, A.; Lutter, R.; Albers, R. Effects of Dietary Supplementation with Carrot-Derived Rhamnogalacturonan-I (CRG-I) on Accelerated Protective Immune Responses and Quality of Life in Healthy Volunteers Challenged with Rhinovirus in a Randomized Trial. Nutrients 2022, 14, 4258, doi:10.3390/nu14204258.
- Abbeele, P.V. den; Deyaert, S.; Albers, R.; Baudot, A.; Mercenier, A. Carrot RG-I Reduces Interindividual Differences between 24 Adults through Consistent Effects on Gut Microbiota Composition and Function Ex Vivo. Nutrients 2023, 15, 2090, doi:10.3390/nu15092090.
- Mercenier, A.; Vu, L.D.; Poppe, J.; Albers, R.; McKay, S.; Abbeele, P.V. den Carrot-Derived Rhamnogalacturonan-I Consistently Increases the Microbial Production of Health-Promoting Indole-3-Propionic Acid Ex Vivo. Metabolites 2024, 14, 722, doi:10.3390/metabo14120722.
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369, doi:10.3390/microorganisms11102369.
- Dahal, R.H.; Kim, S.; Kim, Y.K.; Kim, E.S.; Kim, J. Insight into Gut Dysbiosis of Patients with Inflammatory Bowel Disease and Ischemic Colitis. Front. Microbiol. 2023, 14, 1174832, doi:10.3389/fmicb.2023.1174832.
- Flannigan, K.L.; Nieves, K.M.; Szczepanski, H.E.; Serra, A.; Lee, J.W.; Alston, L.A.; Ramay, H.; Mani, S.; Hirota, S.A. The Pregnane X Receptor and Indole-3-Propionic Acid Shape the Intestinal Mesenchyme to Restrain Inflammation and Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 765–795, doi:10.1016/j.jcmgh.2022.10.014.
- Conn, K.A.; Borsom, E.M.; Cope, E.K. Implications of Microbe-Derived ɣ-Aminobutyric Acid (GABA) in Gut and Brain Barrier Integrity and GABAergic Signaling in Alzheimer’s Disease. Gut Microbes 2024, 16, 2371950, doi:10.1080/19490976.2024.2371950.
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148, doi:10.1016/j.jcmgh.2018.04.003.
- Jia, M.; Fan, Y.; Ma, Q.; Yang, D.; Wang, Y.; He, X.; Zhao, B.; Zhan, X.; Qi, Z.; Ren, Y.; et al. Gut Microbiota Dysbiosis Promotes Cognitive Impairment via Bile Acid Metabolism in Major Depressive Disorder. Transl. Psychiatry 2024, 14, 503, doi:10.1038/s41398-024-03211-4.
- Hul, M.V.; Cani, P.D.; Petitfils, C.; Vos, W.M.D.; Tilg, H.; El-Omar, E.M. What Defines a Healthy Gut Microbiome? Gut 2024, 73, 1893–1908, doi:10.1136/gutjnl-2024-333378.
- Camilleri, M. Leaky Gut: Mechanisms, Measurement and Clinical Implications in Humans. Gut 2019, 68, 1516, doi:10.1136/gutjnl-2019-318427.
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an Emerging Neurometabolic Facet of the Gut Microbiome? Front. Microbiol. 2023, 14, 1098412, doi:10.3389/fmicb.2023.1098412.
- Cutler, A.J.; Mattingly, G.W.; Maletic, V. Understanding the Mechanism of Action and Clinical Effects of Neuroactive Steroids and GABAergic Compounds in Major Depressive Disorder. Transl. Psychiatry 2023, 13, 228, doi:10.1038/s41398-023-02514-2.
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as Essential Mediators in the Gut-Brain Axis. Their Role in Alzheimer’s Disease. Neurobiol. Dis. 2021, 156, 105403, doi:10.1016/j.nbd.2021.105403.
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The Gut Metabolite Indole-3 Propionate Promotes Nerve Regeneration and Repair. Nature 2022, 585–592.
- Kim, C.-S.; Jung, S.; Hwang, G.-S.; Shin, D.-M. Gut Microbiota Indole-3-Propionic Acid Mediates Neuroprotective Effect of Probiotic Consumption in Healthy Elderly: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial and in Vitro Study. Clin. Nutr. 2023, 42, 1025–1033, doi:10.1016/j.clnu.2023.04.001.
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Méd. Sci. 2021, 9, 8, doi:10.3390/medsci9010008.
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42, doi:10.1186/s13073-016-0303-2.
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The Gut Microbiome: A Core Regulator of Metabolism. J. Endocrinol. 2023, 256, e220111, doi:10.1530/joe-22-0111.
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210, doi:10.1152/physrev.00041.2018.
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma Microbiome-Modulated Indole- and Phenyl-Derived Metabolites Associate with Advanced Atherosclerosis and Postoperative Outcomes. J. Vasc. Surg. 2017, 68, 1552-1562.e7, doi:10.1016/j.jvs.2017.09.029.
- Menni, C.; Hernandez, M.M.; Vital, M.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Circulating Levels of the Anti-Oxidant Indoleproprionic Acid Are Associated with Higher Gut Microbiome Diversity. Gut Microbes 2019, 10, 688–695, doi:10.1080/19490976.2019.1586038.
- Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The Mechanism Underlying the Influence of Indole-3-Propionic Acid: A Relevance to Metabolic Disorders. Front. Endocrinol. 2022, 13, 841703, doi:10.3389/fendo.2022.841703.
- Park, S.; Oh, S.; Kim, N.; Kim, E. HMBA Ameliorates Obesity by MYH9‐ and ACTG1‐dependent Regulation of Hypothalamic Neuropeptides. EMBO Mol. Med. 2023, 15, EMMM202318024, doi:10.15252/emmm.202318024.
- Perdijk, O.; Azzoni, R.; Marsland, B.J. The Microbiome: An Integral Player in Immune Homeostasis and Inflammation in the Respiratory Tract. Physiol. Rev. 2024, 104, 835–879, doi:10.1152/physrev.00020.2023.
- Rowland, S.N.; Green, C.G.; Halliwill, J.R.; Singanayagam, A.; Heaney, L.M. Gut Feelings on Short-Chain Fatty Acids to Regulate Respiratory Health. Trends Endocrinol. Metab. 2025, doi:10.1016/j.tem.2024.12.007.
- Woodall, C.A.; McGeoch, L.J.; Hay, A.D.; Hammond, A. Respiratory Tract Infections and Gut Microbiome Modifications: A Systematic Review. PLoS ONE 2022, 17, e0262057, doi:10.1371/journal.pone.0262057.
- Perdijk, O.; Butler, A.; Macowan, M.; Chatzis, R.; Bulanda, E.; Grant, R.D.; Harris, N.L.; Wypych, T.P.; Marsland, B.J. Antibiotic-Driven Dysbiosis in Early Life Disrupts Indole-3-Propionic Acid Production and Exacerbates Allergic Airway Inflammation in Adulthood. Immunity 2024, 57, 1939-1954.e7, doi:10.1016/j.immuni.2024.06.010.
- Liu, C.; Makrinioti, H.; Saglani, S.; Bowman, M.; Lin, L.-L.; Camargo, C.A.; Hasegawa, K.; Zhu, Z. Microbial Dysbiosis and Childhood Asthma Development: Integrated Role of the Airway and Gut Microbiome, Environmental Exposures, and Host Metabolic and Immune Response. Front. Immunol. 2022, 13, 1028209, doi:10.3389/fimmu.2022.1028209.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522, doi:10.1038/s41569-018-0064-2.
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-Propionic Acid Inhibits Gut Dysbiosis and Endotoxin Leakage to Attenuate Steatohepatitis in Rats. Exp. Mol. Med. 2019, 51, 1–14, doi:10.1038/s12276-019-0304-5.
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438, doi:10.1038/nm.4222.
- Portincasa, P.; Bonfrate, L.; Vacca, M.; Angelis, M.D.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Ciaula, A.D. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105, doi:10.3390/ijms23031105.
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379, doi:10.3390/ijms25137379.
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020, 86, 108484, doi:10.1016/j.jnutbio.2020.108484.
- Duan, J.; Dong, W.; Wang, G.; Xiu, W.; Pu, G.; Xu, J.; Ye, C.; Zhang, X.; Zhu, Y.; Wang, C. Senescence-Associated 13-HODE Production Promotes Age-Related Liver Steatosis by Directly Inhibiting Catalase Activity. Nat. Commun. 2023, 14, 8151, doi:10.1038/s41467-023-44026-z.
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Singh, S.; Leishangthem, G.D.; Aich, J.; Kumar, M.; Khanna, K.; Singh, V.P.; et al. Linoleic Acid Metabolite Drives Severe Asthma by Causing Airway Epithelial Injury. Sci. Rep. 2013, 3, 1349, doi:10.1038/srep01349.




