KEYWORDS
Bowel habit
Gut motility
Probiotics
Functional constipation
Irritable bowel syndrome
Abstract
Gut motility is central to bowel function, and dysmotility leads to slow transit and constipation, which is associated with primary gastrointestinal and extraintestinal disorders. Gut transit time, stool frequency, consistency, and bloating are the main clinical outcomes of interest for dysmotility in constipation and are affected by age, gender, lifestyle, and medication use. Although dietary and lifestyle modifications and several lines of pharmacological treatments have been used to treat constipation, new long-term therapeutic solutions are needed to improve treatment satisfaction. To this end, the efficacy of probiotics in the management of constipation has varied. Future randomized controlled trials should thus be based on well-defined probiotic preparations with at least 2 trials, designed, and reported according to unified global standards.
Introduction
Bowel function is central to overall health, and a dysfunctional bowel can lead to constipation or diarrhea, both of which are often observed in functional gastrointestinal disorders (FGIDs), such as functional constipation (FC) and irritable bowel syndrome (IBS). These disorders can overlap and have a high global prevalence (approximately 14% and 10%, respectively), and their management represents a growing public health burden on the clinical and community levels (1, 2).
In addition to FGIDs, which are minor gastrointestinal (GI) ailments without organic causes, other GI or extraintestinal disorders and diseases can present as GI symptoms and dysmotility. For example, certain mood disorders, gastroesophageal reflux disease (GERD), inflammatory bowel disease (IBD), diabetes mellitus, and Parkinson’s disease have been suggested to be associated with dysmotility (1, 2, 3). Thus, improving bowel habits in constipation and diarrhea is fundamental to gut health and general well-being and constitutes an important target for therapeutics or functional foods, including probiotics and prebiotics.
Probiotics are defined as live microbes that, when administered in adequate amounts, confer a health benefit on the host (4). The regulation of intestinal transit, in diarrhea and constipation, is a common function of probiotics (4). Probiotics may prevent antibiotic-associated diarrhea dose-dependently and strain-dependently, whereas there are limited data for probiotics in treating diarrhea-dominant IBS or acute infectious diarrhea (5,6,7). Here, we discuss the clinical endpoints and factors of constipation and its management with probiotics.
Clinical endpoints of constipation
Functional constipation or chronic constipation can be divided into slow-transit constipation (STC), normal-transit constipation (NTC), and rectal evacuation disorders, of which NTC is the most frequent (65%) (8). Patients with STC often have lower rates of high-amplitude propagated contractions after meals, whereas those with NTC have normal rates of bowel movements, but often secondary to perceived difficulty with defecation and hard stools (1).
Bowel habit is defined by the quantity and characteristics of stool that is passed, which are controlled primarily by diet and gut motility. There is no “gold standard” for examining gut motility, although transit time has been evaluated for clinical and research purposes using various techniques (3). Per the Rome IV criteria, the main factor for differentiating FC from constipation-predominant IBS (IBS-C) is the presence and frequency of abdominal pain (1, 2).
Gut transit does not necessarily equate to bowel movement, which has been observed in rectal evacuation disorders. Studies have shown a better correlation between colon transit time (CTT) with stool consistency than with stool frequency. For instance, in assessing chronic constipation, Camilleri et al. (2017) concluded that bowel movement frequency (BMF) alone is not a reliable indicator of delayed colon transit, whereas stool consistency correlated better with it. However, in other studies, BMF was found to be negatively associated with CTT, whereas stool shape is positively linked to higher CTT (3). In healthy adults, BMF has long been considered to be 3-21 bowel movements per week (9). Assessing CTT often needs to be performed in certified clinics on several consecutive days, which is expensive and demanding for patients and volunteers and can result in high drop-out rates and low compliance. Thus, CTT has not always been considered a clinical endpoint in constipation studies.

Influencing factors
Except for the effects from underlying diseases, gut motility is influenced by various factors, such as age, gender, lifestyle, and medication use, which can contribute to constipation.
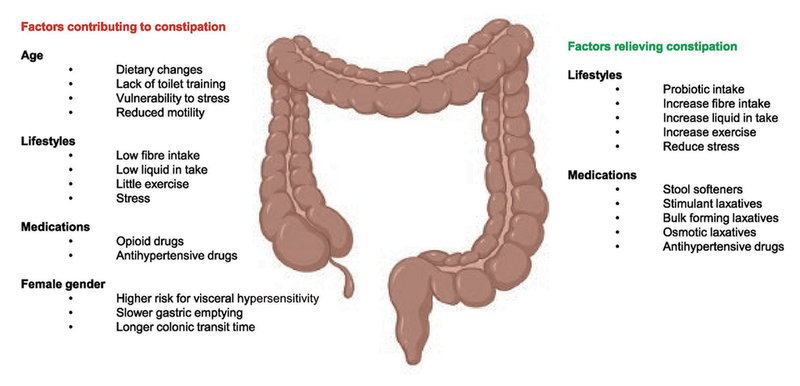
Figure 1. Schematic of main influencing factors of constipation.
©Arthur Ouwehand/IFF; prepared with Biorender.
Age
Functional constipation affects approximately 1 in 10 children worldwide and can continue into adulthood (10). In elderly people, gastrointestinal functions may be impaired with clinically relevant effects. With aging, intestinal motility declines, which can delay digestion and cause constipation. This hypothesis reflects the increasing global prevalence of constipation in the aging population (8, 10).
Gender
Healthy women and men have different visceral sensitivity responses to acute stress, wherein women experience slower gastric emptying and colon transit, increasing their prevalence of GI complaints, such as constipation, diarrhea, and bloating in IBS patients (11). CTT in females is nearly 50% longer than in males (3). However, there is no evidence for gender effects on childhood constipation (10).
Lifestyle
The high incidence of constipation disorders, such as FC and IBS-C, particularly in industrialized countries, is often driven in part by improvements in living standards, which result in behavioral changes, such as an increasingly sedentary lifestyle and reduced fiber consumption, as with low-fiber diets, including the low FODMAP diet (low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) (1, 10). Further, a longer CTT has also been reported in smokers versus nonsmokers but is not associated with coffee or alcohol consumption (3). Undigested food that has accumulated in the GI tract due to a longer CTT has been hypothesized to be the main factor of GI symptoms, such as bloating. In addition, psychological stress induces bowel dysfunction, such as IBS-C in adults (12), whereas daily exposure to stress at home or in school has been linked to constipation in childhood and adolescence (13).
Medications
Medications that are used for systemic disease or local pathology in the colon, such as Parkinson’s disease and colon cancer, respectively, could affect chronic constipation. These medications include, but are not limited to, opioids and antihypertensive agents (1). Opioids can have effects throughout the gastrointestinal tract, including the colon, inducing constipation even at low doses by increasing the absorption of fluids and inhibiting motility (14).
Management and treatment
Modification of lifestyle with adequate fiber and fluid intake and physical activity is the first-line management of constipation, which has generated consistent findings in IBS-C and FC in adults (1, 10). In several systematic reviews, fiber supplementation has had benefits on constipation, given that most adults fail to consume the daily recommended amount of fiber of 14 g/1000 kcal (10). Further, alterations to diets with restrictions on the consumption of poorly absorbable carbohydrates, as in the FODMAP diet, can be helpful in adults and children with IBS-C, but the evidence is not strong, and their effects have not been examined in FC (1, 10).
Laxatives are often used for more severe or refractory constipation. Traditional pharmacological treatments contain osmotic laxatives and stimulant laxatives, such as polyethylene glycol (PEG) and bisacodyl respectively (10). Novel therapeutics, including prosecretory agents (linaclotide, lubiprostone, and plecanatide), serotonergic agents (prucalopride/5-hydroxytryptamine 4 agonist), cholinesterase inhibitors (pyridostigmine), and bile acid (BA) regulators (elobixibat), can alleviate symptoms of constipation by promoting the uptake of fluid in the colon and enhancing GI motility. However, long-term use of laxatives and drugs is often associated with GI complaints, such as nausea and flatulence (with stimulant laxatives), and high-dose PEG correlates with an increased risk of fecal incontinence (8).
Childhood constipation often results in disparate and low responsiveness compared with adult constipation, perhaps due to its different pathophysiology at young age (10), requiring more support from parents, such as biofeedback and toilet training, in addition to common treatment strategies for adults.
Overall, due to the slow responsiveness, dissatisfaction with current treatments, and GI complaints with chronic use, alternative therapeutic solutions for long-term use are needed for constipation, such as probiotics (8, 10).
Microbiota and constipation
Dysbiosis in the gut microbiota has been reported as an important factor in FC and a hallmark of IBS-C, affecting gut motility—likely bidirectionally—and might contribute to its pathogenesis through several mechanisms, mediated by microbial metabolites, including BAs, SCFAs, 5-hydroxytryptamine (serotonin, 5-HT), and methane (8,15). Therapeutic solutions that target the gut microbiota in FC and IBS-C, such as probiotics and prebiotics, aim to restore the homeostasis of gut function by modulating the microbiota at the compositional and functional levels.
Probiotics and constipation
Probiotics have long been used in fermented foods, and several commercial strains have beneficial effects on people with slow transit in FC and IBS-C, such as Bifidobacterium animalis subsp. lactis DN-173010 (16, 17, 18), B. lactis BB-12 (19), and B. lactis HN019 (20). Several meta-analyses reported that probiotics can reduce CTT by 12.4 to 15 hours, increase BMF by 0.8 to 1.3 per week, and improve stool consistency and symptomology in FC and IBS-C in adults (21, 22, 23, 24); however, strain specificity was observed. For instance, B. lactis HN019, but not Lacticaseibacillus casei Shirota, improved BMF and stool consistency (21); these benefits were not observed for a combination of B. lactis strains, either (22). Nevertheless, improvements in bowel movement, transit time, and stool consistency were seen in (21, 22, 23, 24). In contrast, recent meta-analyses have suggested that probiotics have no benefit in the treatment of childhood constipation or other functional assessments (25, 26).
Meta-analyses have highlighted the challenges with studies on probiotics and constipation. Randomized controlled trials (RCTs) on the effects of probiotics for the management of constipation form the core of their innovation to ensure reliable and transparent research. Unfortunately, such trials are rare. Further, current RCTs vary substantially in study design (with regard to strain, dosage, and duration), study population, diagnostic criteria, and reported outcomes, resulting in publication bias and inconsistent efficacies in meta-analyses. Finally, most meta-analyses have pooled data from various strains, which does not allow the estimation of the effect size for a specific probiotic strain. In addition, various multistrain probiotic supplements have been included in such meta-analyses. Bifidobacteria and lactobacilliremain the 2 most commonly used genera, one or both of which have been included in studies that have administered a multistrain probiotic product.
Further trials are needed for probiotic strains (combinations) that have been supported by a single trial. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the American Gastroenterological Association (AGA) recommend that efficacy should be determined with at least 2 RCTs for identical probiotic strains (27). Moreover, there is an urgent need to conduct RCTs per standardized diagnostic criteria to minimize heterogeneity—for example, the greater efficacy of BMF in FC that is diagnosed by the Rome criteria versus a non-Rome-based diagnosis (24). In addition, the use of acidified or fermented milk as a delivery vehicle might create synergies with the probiotics that cannot be compensated for by the placebo in the same matrix. Thus, capsule or granule forms should be advocated to confirm the efficacy of the probiotic alone (27). Because most probiotic strains have not undergone confirmatory trials, future research should focus on complementing this gap. Notwithstanding this need, meta-analyses have suggested a moderate overall benefit of probiotics in the management of constipation.
To date, several mechanisms have been proposed for the effects of probiotics on gut motility: modulate the gut microbiota composition to effect a higher ratio of lactobacilli and bifidobacteria to gram-negative bacteria; produce bacteria-derived microvesicles (MVs), such as by L. reuteri DSM 17938, L. rhamnosus JB-1, B. lactis HN019, and L. rhamnosus GG; and produce or induce the synthesis of neurotransmitters and receptor modulators from endogenous bacteria or food bacteria, such as 5-HT, SCFAs, BAs, and γ-aminobutyric acid (GABA). These MVs, neurotransmitters, and receptor modulators could activate receptors on both sides of the epithelial cell layer to interact with afferent neurons and modulate gut motility, as reviewed in (20, 28). Generally, probiotics cause minor modifications or none at all in the gut microbiota of healthy subjects; nevertheless, they appear to be involved in complex interactions, which remain poorly understood.
Conclusions
Constipation is one of the most common gastrointestinal conditions worldwide, although it is associated with a more affluent lifestyle. Lifestyle changes, such as increased fiber and fluid intake, and exercise are the initial recommendations to resolve constipation. Although efficacious medications exist for constipation, they have several disadvantages due to side effects, providing an opening for alternative remedies, such as probiotics. Meta-analyses suggest a modest benefit for probiotics overall. However, there is a need for repeat studies on strains and strain combinations to document their efficacy and specific mechanism of action.
References and notes
- Camilleri M., Ford A.C., et al., Nat Rev Dis Primers, 3(1), 1-19 (2017).
- Black C.J., and Ford A.C., Nat Rev Gastroenterol Hepatol, 17(8), 473-486 (2020).
- Bohlin J., Dahlin E., et al., Acta Radiol Open, 7(10), 2058460118807232 (2018).
- Hill C., Guarner F., et al., Nat Rev Gastroenterol Hepatol, 11, 506-514 (2014).
- Collinson S., Deans A.,et al., Cochrane Database Syst Rev, 12(12), CD003048 (2020).
- Goodman C., Keating G.,et al., BMJ Open, 11(8), e043054 (2021).
- Wang Y., Chen N., et al., Int J Colorectal Dis, 37(11), 2263-2276 (2022).
- Zhang S., Wang R., et al., Gastroenterol Rep (Oxf), 9(5), 392-401 (2021).
- Salminen S., Bouley C., et al., Br J Nutr, 80(Suppl 1), S147-71(1998).
- Vriesman M.H., Koppen I.J.N., et al., Nat Rev Gastroenterol Hepatol, 17(1), 21-39 (2020).
- Narayanan S.P., Anderson B., et al., Mayo Clin Proc, 96(4), 1071-1089(2021).
- Chang Y.M., El-Zaatari M., et al., Expert Rev Gastroenterol Hepatol, 8(6), 583-585 (2014).
- Liyanarachchi H., Rajindrajith S., et al., Neurogastroenterol Motil, 34(4), e14231 (2022).
- Camilleri M., Lembo A., et al., Clin Gastroenterol Hepatol, 15(9), 1338-1349(2017).
- Rodino-Janeiro B.K., Vicario M., et al., Adv Ther, 35(3), 289-310(2018).
- Agrawal A., Houghton L.A., et al., Aliment Pharmacol Ther, 29(1), 104-114 (2009).
- Marteau P., Cuillerier E., et al., Aliment Pharmacol Ther, 16(3), 587-593(2002).
- Guyonnet D., Schlumberger A., et al., Br J Nutr, 102(11), 1654-1662(2009).
- Eskesen D., Jespersen L., et al., Br J Nutr, 114(10), 1638-1646 (2015).
- Cheng J., Laitila A., et al., Front Nutr, 8, 790561(2021).
- Dimidi E., Christodoulides S., et al., Am J Clin Nutr, 100(4), 1075-1084(2014).
- Zhang C., Jiang J., et al., Clin Nutr, 39(10), 2960-2969(2020).
- Wen Y., Li J., et al., Int J Surg, 79, 111-119(2020).
- Miller L.E., Ouwehand A.C., et al., Ann Gastroenterol, 30(6), 629-639(2017).
- Jin L., Deng L., et al., Medicine (Baltimore), 97(39), e12174(2018).
- Wegh C.A.M., Baaleman D.F., et al., J Pediatr, 240, 136-149.e5(2022).
- McFarland L.V., Karakan T., et al., EClinicalMedicine, 41, 101154(2021).
- Dalziel J.E., Spencer N.J., et al., Int J Biochem Cell Biol, 134, 105963(2021).




