PROBIOTICS:
MICROBES FOR
OPTIMISING HEALTH*
PROBIOTICS

There appears to be a never-ending flow of information about the human microbiota. Reports about new findings on the role of our, mainly intestinal, microbes in health and disease are published on an almost weekly basis in the mainstream media. Diet is obviously a major influence on the composition and activity of the intestinal microbiota. However, there are more specific ways to augment the microbiota; with probiotics (1) and prebiotics (2).
Here we will discuss the benefits of probiotics and the requirements for probiotics.
INTRODUCTION
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (1). While this definition appears clear at first glance, the term “probiotic(s)” is often misused, which has led to widespread confusion as to what is, and what is not, a probiotic. The word “live” indicates that a sufficient quantity (i.e., adequate dose) of the microbe(s) must remain viable in the product during the entirety of shelf life; viability is typically measured and reported as colony-forming units (CFU). The term “microorganisms” can refer to both bacteria and yeasts as well as a single microbial strain or a blend of microbial strains representing different species. The identification of the strain(s) should be performed according to molecular techniques.
The most common commercially available probiotics are members of the genera Bifidobacterium and Lactobacillus (3), and genera formerly known as Lactobacillus (4) and to a lesser, but by no means insignificant, extent Saccharomyces (5) and Bacillus (6) strains. The word “administered” is most commonly interpreted as oral consumption but allows for other possible routes as well, e.g. topical or vaginal. “Adequate amounts” implies sufficient dose for the target health benefit and is normally reported as CFU/capsule/day or CFU/gram/day and is thus linked back to the “live” concept in the definition. It is often recommended that the dose is at least 109 live microbes CFU (7). “Health benefit” is possibly the most challenging term in this definition as health tends to be relative and not fixed. The definition implies that the microbial product must be supported by scientific evidence demonstrating an improvement in a measurable outcome in the target host (e.g. clinical evidence for humans).
“Host” is commonly interpreted as humans but can equally refer to companion animals or livestock.
The definition does not attempt to limit the origin of isolation of the microbes nor does it imply any specific mechanistic requirements such as colonization or adhesion. Although not included in the definition, it is obvious that the microorganisms should be safe for their intended use. These probiotic criteria were more thoroughly described in a recent paper by experts from the International Scientific Association for Probiotics and Prebiotics (ISAPP) and the International Probiotic Association (IPA) Europe (8).
How do you read the label to choose a good quality probiotic product? First, the label should indicate the genus, species and strain for each probiotic organism in the product; e.g. Lactobacillus acidophilus ABC123 (9). The strain is especially important as it guarantees the composition of the product and links the specific microbe to research validating its designation as a probiotic. The dose should be indicated as well as the amount in CFU/capsule or gram and be guaranteed until the expiry date (9). Counts at date of manufacturing are misleading as viability decreases during storage and naturally goes down over time.
The expiry date should therefore also be indicated, in addition to recommended storage conditions. Proper storage ensures the quality and counts during shelf life. Contact details of the manufacturer and recommended use should be indicated too (9). These criteria are stipulated in the IPA-CRN Best Practices Guidelines and ensure a good quality product (10).
The accepted definition implies that a probiotic must be a defined entity, and not an undefined mixture, to allow for appropriate identification to the strain level (11). Although some shared biological mechanisms of action exist among probiotic strains, strain-specific benefits are the presumption unless scientific evidence supports otherwise. Beyond supporting general gastrointestinal health, it is scientifically well recognized that probiotics are strain-, dose-, and health condition-specific (1).
Strains of the same microbial species can substantially differ both genetically (12) and functionally as demonstrated in many cell, animal, and human studies. Thus, clinical evidence is only applicable to the specific probiotic product investigated because different strain(s) and lower dosages may fail to achieve efficacy for the same health outcome. It should, however, be noted that in some jurisdictions organisms can be referred to as probiotics when they belong to a species with a recognised health benefit in that specific jurisdiction (8).
Confusion arises when we look at traditional and more recent exotic sources of microbes; Table 1. A fermented food or beverage (15) is made by extensive microbial growth and many fermented foods and beverages retain live active cultures, although some may undergo further processing which kills the cultures. In general, these cultures were selected for their fermentation properties and added to initiate the fermentation, or were already present in the starting material, enriched during fermentation, not identified to strain level and of unknown dose. Whereas when probiotics are added to fermented foods, it is rarely to provide a technological function, but rather to provide a health benefit from appropriate dosages. This is not to say that fermented foods, FMT, candidate probiotics, and dead microbes do not have the potential to benefit human health (15), but simply that they do not meet the criteria to be termed probiotics (11).
WHAT IS A PROBIOTIC AND WHAT NOT?
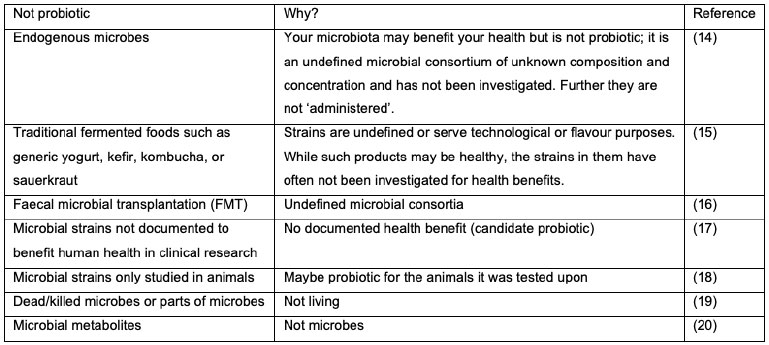
Table 1. What are not probiotics for humans and why (13).
The breadth of probiotic clinical research has expanded dramatically in the last decade to cover virtually every organ system in the human body. Enhanced understanding of the critical role played by the enteric microbiome in health and a disparate range of health conditions has undoubtedly been the impetus for this. The greatest bulk and quality of evidence remains in gastrointestinal and immune indications, such as irritable bowel syndrome, antibiotic associated diarrhoea, and respiratory health, Table 2. However, there are several emerging areas of research including, metabolic, skin, cardiovascular, neuropsychiatric conditions, and vaginal and female urinary tract health (impacting pregnancy and birth outcomes), among others. Although the current body of clinical evidence in a number of these areas limited is, and the responsible microbial mechanisms of action remain to be sufficiently characterised (21). Notwithstanding what is summarised in Table 2, strain (combinations) would need to be tested for their ability to elicit the specific health benefits. However, the potential probiotics hold to augment the microbiome and offer low risk options for unmet medical needs continues to stimulate global research interests. Continued investment in the examination of probiotic efficacy through robustly designed and conducted randomised controlled trials will provide the level of evidence required by healthcare practitioners to fully incorporate probiotics into everyday practice. As mentioned earlier, although we here refer to probiotics as a general descriptor, health benefits tend to be strain specific and thus need to be studied for specific strains and strain combinations.
HEALTH BENEFITS OF PROBIOTICS
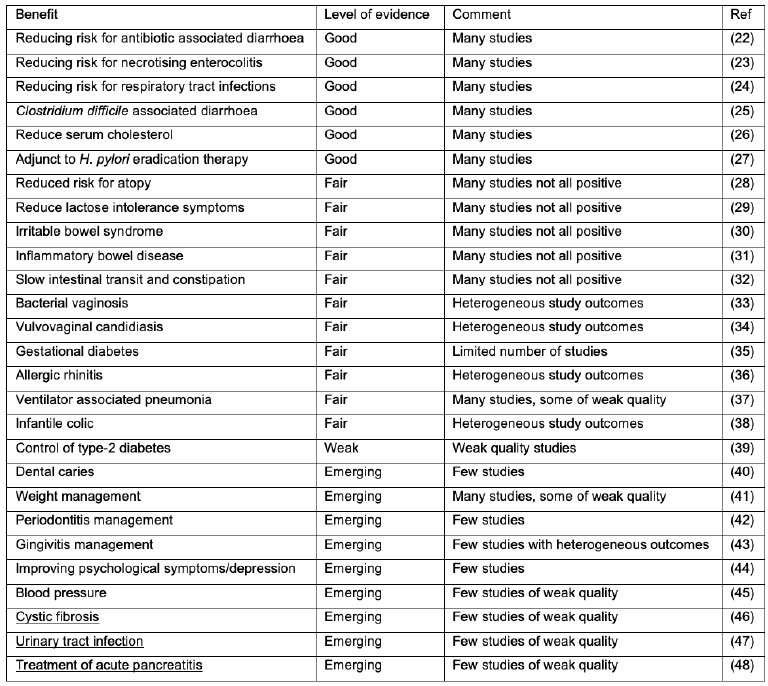
Table 2. Examples of health benefits suggested for probiotics. Note that the table is a generalization, based on meta-analyses. Probiotic products and strains still need to be documented to provide a specific health benefit. Level of evidence is based mainly on the number of studies and the quality of those studies for a particular health benefit.
Probiotics are well defined, and the interpretation of the definition has been further explained in a recent publication (8): proper identification, safety, at least one successful clinical study in humans (or a regulation recognising health benenfit on species level) and appropriate dose at end of shelf-life. Products containing undefined and unstudied microbes, microbial lysates or metabolites produced by microbes may influence human health; however, such products do not meet the criteria to be defined as probiotic.
While probiotics are evidenced to influence disease outcomes and disease risk, they are not drugs. They neither prevent diseases, nor do they cure them. This does not mean that there may not come a time that a specific probiotic could be classified as a drug.
What does this mean in practice? Would one recommend the use of probiotics, and if so, what product? It is best to use products that have been studied for the health benefit you are targeting. However, with probiotics this evidence may not always be readily available or interpretable by consumers or healthcare practitioners. In which case, the best approach is to rely on products of good quality; products that report the strains and not just the species, the counts at the end of shelf life, etc. Furthermore, probiotics are among the safest dietary supplements with an extremely good safety record (49). The benefits may thus be substantial while the risk and costs (50) are small or negligible.
CONCLUSIONS
The article is written on behalf of the International Probiotic Association (IPA); a global non-profit organization that promotes the safe and efficacious use of probiotics throughout the world. At the time of writing, all authors were members of the IPA Scientific Committee and employed by companies manufacturing or marketing probiotics.
CONFLICT OF INTEREST
*Article previously published in Agro Food Industry Hi Tech 32(2) 2021.
Bio...

Dr. Arthur Ouwehand is Technical Fellow at Global Health & Nutrition Sciences, DuPont Nutrition & Biosciences, Kantvik, Finland. Dr. Ouwehand received his M.Sc. degree (1992) from Wageningen University (Netherlands) and his Ph.D. degree (1996) in microbiology from University of Gothenburg (Sweden). Since 1999 he has functioned as Adjunct Professor at the University of Turku (Finland). Since 2004 he has been working for Danisco; now International Flavors and Fragrances. Dr. Ouwehand is the author of more than 350 journal articles and book chapters on probiotics and prebiotics; and he is the editor of four books on lactic acid bacteria and the intestinal microbiota.
ARTHUR C. OUWEHAND1*,
ASHTON HARPER2, JESSICA A. TER HAAR3,
ANTHONY THOMAS4, THOMAS TOMPKINS5
*Corresponding author
1. International Flavors and Fragrances, Kantvik, Finland
2. ADM Protexin Ltd., Somerset, United Kingdom; present address: Roche Diagnostics Ltd., Burgess Hill, United Kingdom
3. International Probiotic Association, Los Angeles, USA
4. Jarrow Formulas, Los Angeles, USA
5. Lallemand Health Solutions, Montreal, Canada
ARTHUR C. OUWEHAND et al.
International Flavors and Fragrances (IFF) | Finland




