GUT MICROBIOTA DEVELOPMENT
IN EARLY LIFE AND THE ROLE
OF DIET AND PROBIOTICS
FOR LIFELONG HEALTH*
MICROBIOME

Our health is largely defined by the microbiota we harbor in our body beginning with the first breath we take. Communication between the host and co-habiting microbes is complex, and increasing evidence suggests that microbial signals not only affect our health, but also program the future health, especially during the first 1000 days. Several factors, such as urban environment, high hygiene level, small family size, low exposure to animals, antibiotics, formula-feeding as well as birth via Caesarean-section (C-section) can disturb evolutionarily conserved gut microbiota colonization and maturation These alterations in the maturation process may predispose an individual to certain health disadvantages. Modulation of the early gut microbiota development by diet and especially with probiotics are potential means to support an infants’ health and possibly extending later in life. However, more research is warranted on the efficacy of modulators of early microbiota development, including probiotic strains as well as identifying the ideal products for the optimal outcome in various situations.
Microbial programming occurs already in early life when the bacterial signaling molecules induce permanent or long-lasting changes to host cell functioning, resulting in reprogramming of major endocrine axes [1], as well as immune [2], and metabolic health [3], via e.g. epigenetic modifications including DNA methylation [4]. The consequences may persist beyond the disappearance of a particular bacterial species in the gut, causing relatively permanent effects on immune, metabolic and cognitive development, and possibly impacting the risk of various associated conditions such as allergies [5], obesity [3] and autism spectrum disorders [6].
While prenatal microbial programming is still controversial among scientists [7], the neonatal phase can be described as the major “colonization march” in which the “early invaders”, mainly facultative anaerobes, such as Enterobacteriaceae, modify the gut environment to be suitable for the next microbes, “the settlers”, mainly strict anaerobes such as Bifidobacterium spp. [8, 9].
As a consequence of the modernization of the society, alternatives to the evolutionarily conserved practices have been introduced, such as C-section delivery and formula feeding, and recently evidence has increased showing that these practices also alter the colonization march within the newborn gut[10]. Altered early colonization modulates the gut microbiota composition later in infancy, reinforcing the effect of early colonizers on later health [9, 11].
INTRODUCTION
Human evolution can be seen merely as a coevolution of the metagenome composed of not only the human gene complements but also of all associated microbial genes [12]. During thousands of years of human evolution, there has been very little variability in the early life factors, and vaginal birth enriched infant gut with commensal bacteria including Bifidobacterium spp., Bacteroides/Parabacteroides species, and higher abundance of lactobacilli species associated with maternal vaginal microbiota [13], and gut microbiota[14, 15].
The microbes colonizing the infant gut engage into complex interaction with the host cells, including immune and even brain cells [2, 16]. As infants are born with immature immune system, the microbial signals are necessary for guiding the immune system maturation process and the establishment for tolerance [17].
Evolutionarily conserved gut microbiota development pattern
The gut microbiota of vaginally delivered and breastfed infants follows a certain development pattern, which has been highly conserved during the evolutionary development of humans (Fig 1), characterized by reduction of the species diversity from the situation immediately after birth [18]. The very first colonizers of the infant gut are facultative anaerobes, species within bacterial families Enterobacteriaceae, Streptococcaceae, and Staphylococcaceae. Many early colonizers are only transient colonizers of the infant gut and shape the microenvironment in the gut suitable for the next colonizers. The early colonizers are followed by strict anaerobes e.g. Bifidobacterium spp., Bacteroides spp., and clostridia [19-21]. The colonization pattern is relatively conserved and similar in different geographical areas [19-21] although differentiation to certain microbiota types is feasible from global perspective [22]. During the weaning phase, high-fiber foods, increase the prevalence of Firmicutes and Prevotella spp., whereas intake of animal protein increases Bacteroidetes prevalence [23]. After weaning, a more complex microbiota is established with predominant genera being Bifidobacterium, and Bacteroides, and Blautia coccoides, being the predominant species [23]. Geographical differences in the gut microbiota composition during older infancy between the northern and southern European countries suggest that differences in the early weaning diet may have an impact in the gut microbiota development [24].
NORMAL COLONIZATION OF NEWBORN GUT AND EARLY DISRUPTIONS OF GUT MICROBIOTA DEVELOPMENT
Environmental modulators of infant gut microbiota development
The most important factor affecting the gut microbiota colonization is the mode of delivery.Compared to vaginally born babies, C-section born babies are in general depleted of the commensal bacteria and dominated instead by microbes commonly associated with hospital environments such as Enterococcus spp., Staphylococcus epidermis, Streptococcus parasanguinis, Klebsiella spp., Enterobacter cloacae, and Clostridium perfringens [10]. More specifically,after C-section, the infant microbiota is less prevalent in Lactobacillus of vaginal origin [25], as well as Bifidobacterium spp. and Bacteroides spp. of maternal fecal origin [20, 26, 27]. Instead, the infant is more exposed to skin microbes, such as Corynebacterium spp., Propionibacterium spp., and Staphylococcus spp. [13], as well as oral and throat microbes such as Haemophilus spp., Staphylococcus spp., and Veillonella spp. All in all, C-section compromises mother-to-infant microbial transmission reducing the percentage of early colonizers of maternal fecal origin from 72% to 41% [21].
C-section is also linked to antibiotic exposure during birth, which may confound conclusions about the effect of delivery mode to the gut microbiota. Early life antibiotic exposure has been shown to delay microbiota maturation, and cause changes in the gut microbiota composition that vary according to antibiotic type, timing and route of administration, duration of use, underlying conditions, number of exposures to commensal bacteria, and differences between individual subjects [20]. While Intrapartum antibiotic prophylaxis (IAP) is applied to prevent maternal infection after C-section, it affects both the milk microbiota [28], and infant’s intestinal [29-32] and oral [33] microbiota during the first months of life. IAP is also associated with outcomes such as higher incidence of infantile colic [34].
Maternal factors, affect the way the infant gut microbiota is developing in early life [35]. Both animal [36] and human trials [37] indicate that maternal dietary factors such as fiber and fat intake before and during pregnancy modulate the microbial diversity and composition of the infant in a transgenerational manner not fully reversible by infant diet after birth. Additionally, westernized diet seems to result in progressive loss in the microbial diversity [38], while microbial changes caused by maternal obesity or excessive weight gain during pregnancy [39] transgenerationally pass on to infant [40]. Further, insufficient exposure to human milk [41], or excess antibiotic exposure [10] can disrupt the early phases of the gut microbiota development. Human milk contains many microbes that either transiently or more permanently can inhabit the infant gut, including B. bifidum, B. breve, B. longum subsp. infantis, B. longum subsp. longum, B. dentium, B. adolescentis, and Limosilactobacillus fermentum, Lactiplantibacillus plantarum, and Lactobacillus oris [35, 42, 43]. In addition to bacteria, human milk contains oligosaccharides referred to as Human Milk Oligosaccharides (HMOs), prebiotic oligosaccharides that function as an energy source for bacteria that are beneficial to the infant gut microbiota [35], and many bioactive compounds that have central roles as mediators and modulators of both the gut microbiota and host physiological development [44].
All in all, the development and programming of immune, metabolic, and cognitive health of human infant has evolved to proceed optimally in the evolutionarily conserved environment, and can thus be compromised when early life environment and circumstances significantly deviate from those of our ancestors.
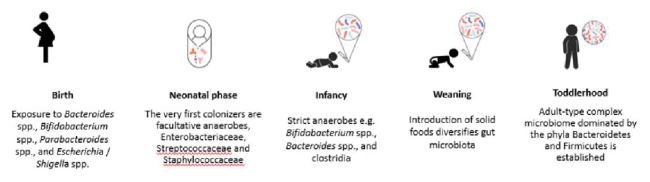
Figure 1. The cascade of gut microbiota colonization of a vaginally born breastfed baby is conserved. Illustration drafted based on Chu et al. 2017, Ferretti et al. 2018, Bokulich et al. 2016, Tanaka et al. 2017 [18-21, 23].
Several probiotic strains exist in market targeted for infants in early life, and the safety/tolerance and efficacy of these strains has been demonstrated in clinical trials [45]. Studies show that probiotics may have the ability to affect beneficially on the infant gut microbiota restoration/development after disruptive event. For instance, a recent study [46] showed that when infants born via C-section received a probiotic combination of Bifidobacterium animalis subsp. lactis HN019, B. animalis subsp. lactis Bi-07, and Lacticaseibacillus rhamnosus HN001 for 28 days, the gut microbiota diversity of these infants were similar to the vaginally born controls.
In a study conducted in rural India with 4556 newborn infants receiving Lactiplantibacillus plantarum 202195 (1B CFU daily) and fructooligosaccharide (150 mg) for 7 days, a significant 42% reduction in the occurrence of sepsis and death as well as lower respiratory infections in the probiotic group was seen when compared with placebo [47]. Also, in a, randomized, double-blind, and placebo-controlled clinical trial with 474 mother-infant pairs receiving L. rhamnosus HN001, B. lactis HN019, or placebo from 35 weeks of pregnancy to 2 years of infant life in New Zealand . a statistically significant reduction in the cumulative prevalence of eczema and allergic sensitization was observed in the children receiving HN001 [48-51] with association between increased L. rhamnosus and higher infant gut microbiota functional capacity to transport glycerol-3 phosphate [52].
As a future guidance, well-powered and designed clinical trials with a long-term follow up are needed. Probiotics could also be combined with HMOs that selectively feed beneficial species, [53]. Furthermore, when selecting probiotic strain(s) especially for restoring the infant gut microbiota development, it would be important to consider specific bacterial species that in an evolutionary conserved situation would inhabit the infant gut and the natural route of administration of these bacteria in, for example vaginal birth [54].
INFANT GUT MICROBIOTA RESTORATION AND IMMUNE SYSTEM DEVELOPMENT
To conclude, the gut microbiota composition evolves as a result of ongoing succession of colonization throughout the early phases of life (Fig 2). During evolution this succession has been extremely conserved, and this succession pattern is linked to acute and later health of the infant. In the future, as research data accumulates on the efficacy and safety of various microbial solutions for infants, it will enable the development of more efficacious products that steer the gut microbiota development back on track in various situations where it has been deviated from the evolutionarily conserved course.
CONCLUSIONS AND FUTURE PERSPECTIVES
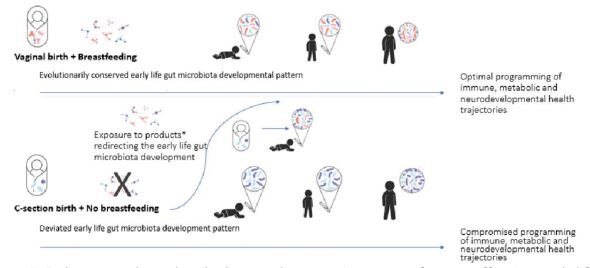
Figure 2. Delivery mode and early diet are the most important factors affecting early life gut microbiota colonization pattern, which has long lasting effect on infant health trajectories.
*Article previously published in Agro Food Industry Hi Tech 32(3) 2021.
KAILANTO HENNA-MARIA et al.
IFF | Finland
Bio...
Dr. Henna-Maria Kailanto is a Senior Scientist at IFF Health devoted to understand the programming effects of early life nutrition on microbiota development and immune, metabolic, and cognitive health. Henna-Maria earned her MSc and PhD degrees in Food Chemistry from the University of Turku. After her PhD, she has applied her expertise in clinical trials to explore human milk components and probiotics as modifiers of infant development and health. Currently, as Technical Lead of Early Life Nutrition Segment she is ambitious to develop new innovative solutions to support early life health.





